
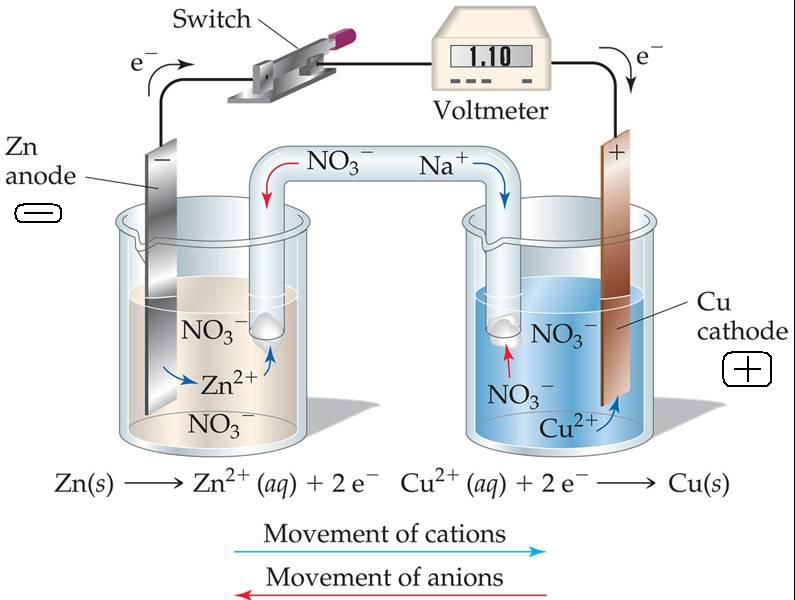
LTO, or Lithium titanate (Li 4 Ti 5 O 12) is a highly stable anode material that is ideally suited for electrode sheets in batteries requiring high c-rates and long life cycles. In a battery, the cathode is always the negative and the anode is always the positive. Lithium Titanate (LTO) Anode Electrode Sheets. The anode is important because the material choices of the anode will affect the safety, charging speed. The electrode that absorbs the electrons emitted in the discharge is called the anode. The anode is the negative electrode in a battery. The electrode that emits electrons when the battery discharges is called the cathode. The results also verify that the root mean square error of the cathode and anode potential estimated by the observer is less than 0.02 V for a charging current range from 0.3C to 1C. Each battery has two electrodes responsible for chemical reactions that provide energy. The results show that the linearised model reduces the operation time by more than 99% compared with the full-order SPMe model using the same processor. If the reactive components of an electrochemical cell are placed in contact with each other. coated in a thin film onto a current collector (typically, an aluminum foil is used with the cathode, and a copper foil for the anode). Contact 1-76 Request a Quote DeltaModTech. The linearised model and the designed observer are validated by the experimental results of a three-electrode cell. An in-depth look at the optimal coating process to use with battery electrodes to improve accuracy and precision. The observer design is based on the model order reduction and linearisation of a single particle model with electrolyte (SPMe) to achieve acceptable accuracy with a low calculation cost. This paper proposes an observer for estimating the cathode and anode potentials based on the reduced-order electrochemical model, which only needs terminal voltage to track the cathode and anode potentials and their internal charge concentration. It is important to monitor the anode potential during battery charging, but it is not practical to measure the inside of the battery directly for a commercial cell. The fast charging of Lithium-ion batteries within electric vehicles can accelerate the side reaction of lithium plating due to an anode potential that occurs as state of charge increases.


 0 kommentar(er)
0 kommentar(er)
